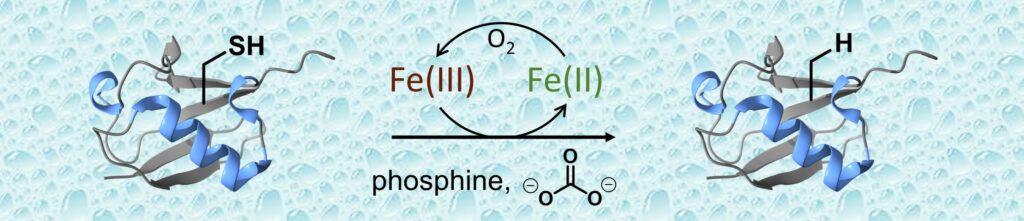
This article describes a novel method for effecting the selective desulfurization of cysteine into alanine at the protein level, using an iron-catalyzed process reminiscent of aquatic chemistry.
One pillar of protein chemical synthesis based on the application of ligation chemistries to cysteine is the group of reactions enabling the selective desulfurization of cysteine residues into alanines. Modern desulfurization reactions use a phosphine as a sink for sulfur under activation conditions involving the generation of sulfur-centered radicals. Here we show that cysteine desulfurization by a phosphine can be effected efficiently by micromolar concentrations of iron under aerobic conditions in hydrogen carbonate buffer, that is using conditions that are reminiscent of iron-catalyzed oxidation phenomena occurring in natural waters. Therefore, our work shows that chemical processes taking place in aquatic systems can be adapted to a chemical reactor for triggering a complex chemoselective transformation at the protein level, while minimizing the resort to harmful chemicals.
Selected as a Hot Paper by the journal.